@Lee_Ann
Hi Lee,
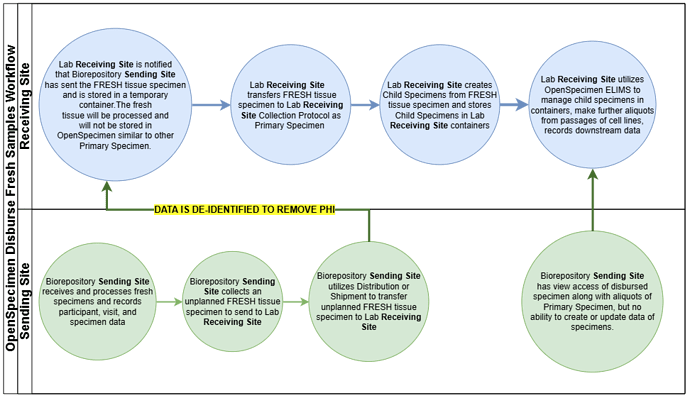
I believe this is a topic we discussed over a year ago as a scenario we were also struggling with. We never did come up with a way to handle it other than distributing specimens from the CP managed by Lab A (the tissue banking IRB). Lab B who holds the Use IRB would have to submit a request for specimens to Lab A. The specimens would then be bulk imported as new specimens into a new CP created for Lab B.
As you stated, this results in the need to establish a relabeling scheme for any scenario where this would occur. Also you lose all linkage to the clinical data associated with that new specimen. And it skews the statistics in terms of the metrics for new specimens that exist in any biorepository or the parent institute.
If a specimen-centric protocol is used to capture the newly distributed specimens for Lab B, the specimen hierarchy is lost along with any other specific longitudinal information captured at the visit level. I’d love a solution for this as it’s been a big blocker for me too.
If a participant-centric protocol is used with no patient info because the specimens are de-identified, then catalogs and queries appear as if there are multiple individuals with the same condition.
I’m thinking out loud now:
If the unique Participant ID (not PPID) could be set to exist, yet be blinded as PHI for some studies, perhaps that’s a solution? A Use IRB CP could exist in which you use the Participant ID as the key, but do not import or display any of the actual PHI fields in the new protocol. The ClinDX fields could be imported along with the visit information and even the specimen hierarchy (could use the same method of the ‘Visit ID’ and the ‘Specimen ID’ for the already existing hierarchy. Then the actual specimen that is created maintains that linkage using the ‘Specimen ID’. Lab B imports using Specimen ID and relabels with new schema?
Or perhaps add a feature similar to the ability to restrict visibility by registration site and instead create visibility by Distribution Site. So if you are associated with DP Site (Lab B), you would have access only to the specimens that have been distributed to you (with or without access to PHI which could vary). Would that be annoying to the staff of Lab A who might not want to see the processed specimens for samples they no longer manage?
Would love to hear more thoughts on this subject,
Donna
Biomedical Informatics Core
University of Utah